RESEARCH FOCUS
Theme #1: Pancreatic beta cell stress responses in type 1 diabetes.
Type 1 Diabetes (T1D) is generally viewed as a disease of the immune system, where beta cells are simply ‘sitting ducks’ that are destroyed due to the phenomenon of autoimmunity (self-targeted immunity). Under this paradigm, the vast majority of clinical trials have focused on ways to prevent or cure this disease by re-establishing a functional immune system or blunting the autoimmune response. Remarkably, this has now led to the first FDA approved immunotherapy for delaying T1D onset (Teplizumab also called Tzield) an anti-CD3 therapy that targets autoimmune T cells.
But what about the beta cells? Are they merely passive victims in the complex dialogue that culminates in T1D? Our work is taking a different perspective as compared with the historical view of T1D as an autoimmune disease in that we are focusing our efforts on understanding how stress responses in beta cells and their interactions with the immune system contribute to disease onset and progression.
But what about the beta cells? Are they merely passive victims in the complex dialogue that culminates in T1D? Our work is taking a different perspective as compared with the historical view of T1D as an autoimmune disease in that we are focusing our efforts on understanding how stress responses in beta cells and their interactions with the immune system contribute to disease onset and progression.
A growing body of evidence indicates that various forms of stress occurring during the development of T1D leads healthy beta cells to adopt dysfunctional states or undergo cell death. Surprisingly, although it was once believed that all beta cells are lost in people living with T1D, recent work is now showing that in the majority of cases, there may be a substantial proportion of beta cells remaining that have become dysfunctional. Immune and metabolic stress leads to beta cell loss by apoptosis (programmed cell death) via the terminal Unfolded Protein Response (UPR). Exciting recent clinical trials have shown that the UPR is a candidate drug target for improving beta cell function after T1D diagnosis. The Type I interferon (IFN) response leads to a hyperexpression of antigen-presenting molecules and eventually sensitizes beta cells to apoptosis. However, in addition to stressed beta cell death pathways, it is now being appreciated that beta cells also undergo non-lethal stress in T1D.
Non-lethal responses may include Senescence and a variety of other dysfunctional states, such as changes in identity (loss of insulin or acquisition of other islet hormones), defects in proinsulin processing and defects in autophagy. The variety of beta cell stress responses may underlie some aspects of the interpersonal variability in T1D (disease heterogeneity). At a cellular level, beta cells themselves are heterogeneous, meaning not all the beta cells in an islet are functionally the same. This phenomenon may also explain the ability of different beta cells to respond differently to the same kind environmental stress. Currently, our knowledge in this emerging field is very limited. The specific environmental triggers and genetic factors that lead beta cells to adopt distinct stress responses en route to T1D and how this relates to the person-to-person variability in T1D development and onset are important questions. Addressing these fundamental questions will be critical for developing new non-immune-based therapies for T1D that can benefit the most people.
Non-lethal responses may include Senescence and a variety of other dysfunctional states, such as changes in identity (loss of insulin or acquisition of other islet hormones), defects in proinsulin processing and defects in autophagy. The variety of beta cell stress responses may underlie some aspects of the interpersonal variability in T1D (disease heterogeneity). At a cellular level, beta cells themselves are heterogeneous, meaning not all the beta cells in an islet are functionally the same. This phenomenon may also explain the ability of different beta cells to respond differently to the same kind environmental stress. Currently, our knowledge in this emerging field is very limited. The specific environmental triggers and genetic factors that lead beta cells to adopt distinct stress responses en route to T1D and how this relates to the person-to-person variability in T1D development and onset are important questions. Addressing these fundamental questions will be critical for developing new non-immune-based therapies for T1D that can benefit the most people.
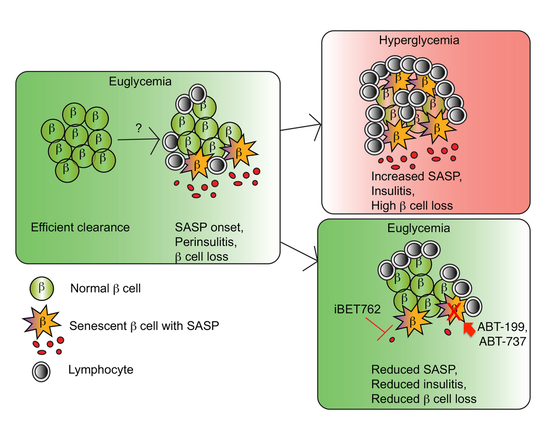
Our work is focused on mechanisms of beta cell senescence in T1D and its interplay with the immune system and other stress responses. During the development of T1D, a subset of beta cells undergo a DNA damage response and enter into a programmed state of cell proliferation arrest (senescence). Once senescent, these beta cells can begin exporting inflammatory chemical messengers that activate the immune system, a process called SASP. The SASP may stimulate the autoimmune response attracting more self-reactive immune cells (lymphocytes) to invade the islet (insulitis) and destroy more beta cells, a vicious cycle that culminates in T1D onset. Remarkably, when these harmful SASP Beta cells are eliminated with senolytic compounds (ABT-737 or ABT-199), or their SASP is suppressed (iBET-762) early on, healthy beta cells may be preserved and T1D averted in a preclinical mouse model. We are currently investigating the mechanisms of senescence in human beta cells, how these senescent beta cells arise in T1D using novel mouse models and the interplay of senescence with other pathways (e.g. UPR and IFN response) as well as with the immune system to develop novel interventions.
In exciting new work recently published (see the publication here), in collaboration with our colleagues at the University of Wisconsin-Madison, we found that senescence is more complex that we thought and it may not always be harmful! Disruption of the UPR mediators Ire1 or Atf6 in a T1D mouse model leads to an early/protective form of senescence that helps the immune system remove these potentially harmful cells early on in the disease. This ultimately slows the rate of autoimmune beta cell loss and prevents diabetes in the mice. Many questions have been raised from this ground-breaking new study and we are actively investigating the beneficial versus deleterious roles of beta cell senescence and its interplay with immune surveillance mechanisms in T1D.
In exciting new work recently published (see the publication here), in collaboration with our colleagues at the University of Wisconsin-Madison, we found that senescence is more complex that we thought and it may not always be harmful! Disruption of the UPR mediators Ire1 or Atf6 in a T1D mouse model leads to an early/protective form of senescence that helps the immune system remove these potentially harmful cells early on in the disease. This ultimately slows the rate of autoimmune beta cell loss and prevents diabetes in the mice. Many questions have been raised from this ground-breaking new study and we are actively investigating the beneficial versus deleterious roles of beta cell senescence and its interplay with immune surveillance mechanisms in T1D.
Theme #2: Extracellular vesicles from beta cells and immune cells in type 1 diabetes.
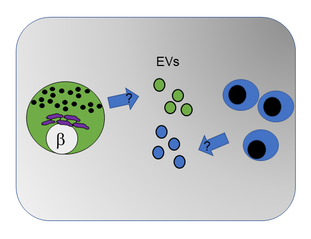
Almost all cells can package and export a subset of their DNA, RNA and/or proteins into small nanoparticle ‘life-rafts’ collectively termed secreted extracellular vesicles (or EVs for short). EVs can be isolated from culture supernatants, blood or other bodily fluids and are emerging as a particularly attractive target for developing new disease biomarkers as well as engineering a means to specifically target the delivery of therapeutics to cells of interest in the body. A secondary theme in our work investigates beta cell EV cargo to understand how EVs communicate signals between islets and immune cells in T1D. We are employing human beta cell culture models, along with innovative mouse genetic models to specifically study beta cell-derived EVs in T1D and understand how EV signaling shapes autoimmunity and disease progression.
Theme #3: Clinical biomarkers of stress and function in surviving beta cells in type 1 diabetes.
Following from our basic/fundamental research on beta cell senescence, we are now launching a pediatric clinical research study to explore novel biomarkers of survivor beta cell stress in recently diagnosed T1D patients. Our clinical study is entitled: "Monitoring markers of survivor beta cell function and stress in recently diagnosed type 1 diabetes".
Is your child interested in participating in a research study on type 1 diabetes?
Dr. Peter Thompson and Dr. Brandy Wicklow are conducting a research study to measure markers of the remaining insulin-producing beta cells in the first 5 years after diagnosis of type 1 diabetes in children and teens at the Children’s Hospital Research Institute of Manitoba and Health Sciences Centre in Winnipeg.
The ‘survivor’ beta cells send out marker signals akin to an S.O.S. distress call. These markers are detectable in blood and can provide useful information about the beta cell’s functional status and stress level. A better understanding of ‘survivor’ beta cells may reveal important clues as to how type 1 diabetes progresses after diagnosis and offer insights that will help us improve glycemic control in recently diagnosed individuals.
Your child may be eligible if they are:
-Aged 2-17.5 years old
-Physician diagnosed type 1 diabetes within 0-5 years
If you would like to receive more information and be contacted about this study, please email the study principal investigator Dr. Thompson ([email protected]) or contact by phone (204-975-7706).
Is your child interested in participating in a research study on type 1 diabetes?
Dr. Peter Thompson and Dr. Brandy Wicklow are conducting a research study to measure markers of the remaining insulin-producing beta cells in the first 5 years after diagnosis of type 1 diabetes in children and teens at the Children’s Hospital Research Institute of Manitoba and Health Sciences Centre in Winnipeg.
The ‘survivor’ beta cells send out marker signals akin to an S.O.S. distress call. These markers are detectable in blood and can provide useful information about the beta cell’s functional status and stress level. A better understanding of ‘survivor’ beta cells may reveal important clues as to how type 1 diabetes progresses after diagnosis and offer insights that will help us improve glycemic control in recently diagnosed individuals.
Your child may be eligible if they are:
-Aged 2-17.5 years old
-Physician diagnosed type 1 diabetes within 0-5 years
If you would like to receive more information and be contacted about this study, please email the study principal investigator Dr. Thompson ([email protected]) or contact by phone (204-975-7706).
Current Projects.
We are currently working on several projects at the intersection of basic/translational islet biology and immunology in T1D, some of which are outlined below. Our projects utilize a variety of traditional and cutting-edge multidisciplinary approaches spanning endocrine physiology, cell biology, genetics, immunology and pharmacology to address fundamental questions about T1D and develop novel approaches to target beta cell dysfunction and the interplay with the immune system. We allow our research questions to determine the specific approaches, tools and collaborative expertise required to arrive at answers. We use a combination of rodent cell lines, the preclinical NOD mouse model for T1D, and parallel studies on human islets and donor pancreas tissue. Trainees in the lab will develop a broad skill set and expertise relevant to a variety of future career goals.
1. Effect of anti-CD3 therapy on senescent beta cells in T1D. Teplizumab is an anti-CD3 monoclonal antibody therapy that was recently approved by the FDA to delay symptomatic T1D in those at risk. As such, it represents the first treatment with clinical efficacy in slowing the progression of the disease - a major advance for the field and families affected by T1D. However, the mechanisms of action of Teplizumab and specifically its impact on beta cell function long-term, remains unclear. In this project we are using studies in NOD mice to model the effect of anti-CD3 monoclonal antibody therapy to understand whether it may impact the development and/or turnover of senescent beta cells.
2. Targeting senescent human beta cells as a novel therapy for T1D. We do not yet know how senescent human beta cells stay alive and persist during the development of T1D. We also lack a basic understanding of specific markers expressed by senescent human beta cells in vivo. Understanding this is important for developing therapies specific to senescent beta cells in people during T1D (as opposed to in our mouse models). In this project, we are systematically interrogating the role of prosurvival proteins and other candidate senescence-associated proteins in human beta cells. We are working with the Network for Pancreatic Organ Donors with Diabetes (nPOD) repository to identify the key prosurvival proteins in senescent beta cells during T1D. We are also using our recently established human donor islet culture model and a human beta cell line model (Brawerman et al. Mol Metab, 2022) to determine the functional importance of these survival pathways and to test novel drug therapies to eliminate senescent human beta cells.
3. Understanding the interplay between senescent beta cells and the immune system in T1D. Senescent beta cells are capable of secreting inflammatory factors and EVs that can modify the immune response in T1D and may drive T1D onset and progression. In this project, we are using the NOD mouse model and studies on human islets and immune cells to explore how senescent beta cells interact with and modify immune responses during T1D, as well as to understand how immune cells may trigger progression of senescence in beta cells.
4. Delineating the effect of senescence on human islet function. Our initial studies suggest that senescence does not trigger major problems in beta cell insulin secretion from adult human islets in culture (Brawerman et al. Mol Metab, 2022), however our studies suggest defects in glucagon secretion as a consequence of senescent beta cells in islets (Brawerman et al. Front Endocrinol, 2022). In this project, we are performing in-depth molecular and metabolic profiling to study how senescence alters the human beta cell transcriptome and metabolic programs and may negatively impact alpha cells.
2. Targeting senescent human beta cells as a novel therapy for T1D. We do not yet know how senescent human beta cells stay alive and persist during the development of T1D. We also lack a basic understanding of specific markers expressed by senescent human beta cells in vivo. Understanding this is important for developing therapies specific to senescent beta cells in people during T1D (as opposed to in our mouse models). In this project, we are systematically interrogating the role of prosurvival proteins and other candidate senescence-associated proteins in human beta cells. We are working with the Network for Pancreatic Organ Donors with Diabetes (nPOD) repository to identify the key prosurvival proteins in senescent beta cells during T1D. We are also using our recently established human donor islet culture model and a human beta cell line model (Brawerman et al. Mol Metab, 2022) to determine the functional importance of these survival pathways and to test novel drug therapies to eliminate senescent human beta cells.
3. Understanding the interplay between senescent beta cells and the immune system in T1D. Senescent beta cells are capable of secreting inflammatory factors and EVs that can modify the immune response in T1D and may drive T1D onset and progression. In this project, we are using the NOD mouse model and studies on human islets and immune cells to explore how senescent beta cells interact with and modify immune responses during T1D, as well as to understand how immune cells may trigger progression of senescence in beta cells.
4. Delineating the effect of senescence on human islet function. Our initial studies suggest that senescence does not trigger major problems in beta cell insulin secretion from adult human islets in culture (Brawerman et al. Mol Metab, 2022), however our studies suggest defects in glucagon secretion as a consequence of senescent beta cells in islets (Brawerman et al. Front Endocrinol, 2022). In this project, we are performing in-depth molecular and metabolic profiling to study how senescence alters the human beta cell transcriptome and metabolic programs and may negatively impact alpha cells.
Selected recent publications from the lab.
Jasmine Manji, Jasmine Pipella, Gabriel Brawerman and Peter J. Thompson. (2024). Exploring transcriptional regulation of beta cell SASP by Brd4-associated proteins and cell cycle control protein p21. Epigenomes PMID:38534794
Nayara Rampazzo Morelli, Jasmine Pipella and Peter J. Thompson. (2024). Establishing evidence for immune surveillance of β-cell senescence. Trends in Endocrinology & Metabolism PMID:38307810
Hugo Lee, Peter J. Thompson and Feyza Engin. (2024). Protocol for quantifying SA-β-gal activity as a measure of senescence in islets of a mouse model of type 1 diabetes. STAR Protocols PMID:38427571
Hugo Lee*, Gulcan Semra Sahin*, Chien-Wen Chen, Shreyash Sonthalia, Sandra Marin Canas, Hulya Zeynep Oktay, Alexander T. Duckworth, Gabriel Brawerman, Peter J. Thompson, Maria Hatzoglou, Decio L. Eizirik and Feyza Engin. (2023). Stress-induced β cell early senescence confers protection against type 1 diabetes. Cell Metabolism PMID: 37949065 *Equally contributing authors.
Peter J. Thompson, Jasmine Pipella, Guy A. Rutter, Herbert Y. Gaisano and Pere Santamaria. (2023). Islet autoimmunity in human type 1 diabetes: initiation and progression from the perspective of the beta cell. Diabetologia PMID:37488322
Gabriel Brawerman, Vasilis Ntranos and Peter J. Thompson. (2022). Alpha cell dysfunction in type 1 diabetes is independent of a senescence program. Frontiers in Endocrinology PMID:36277717
Gabriel Brawerman, Jasmine Pipella and Peter J. Thompson. (2022). DNA damage to β cells in culture recapitulates features of senescent β cells that accumulate in Type 1 Diabetes. Molecular Metabolism PMID:35660116
See the full list of Dr. Thompson's peer-reviewed journal publications here.
Nayara Rampazzo Morelli, Jasmine Pipella and Peter J. Thompson. (2024). Establishing evidence for immune surveillance of β-cell senescence. Trends in Endocrinology & Metabolism PMID:38307810
Hugo Lee, Peter J. Thompson and Feyza Engin. (2024). Protocol for quantifying SA-β-gal activity as a measure of senescence in islets of a mouse model of type 1 diabetes. STAR Protocols PMID:38427571
Hugo Lee*, Gulcan Semra Sahin*, Chien-Wen Chen, Shreyash Sonthalia, Sandra Marin Canas, Hulya Zeynep Oktay, Alexander T. Duckworth, Gabriel Brawerman, Peter J. Thompson, Maria Hatzoglou, Decio L. Eizirik and Feyza Engin. (2023). Stress-induced β cell early senescence confers protection against type 1 diabetes. Cell Metabolism PMID: 37949065 *Equally contributing authors.
Peter J. Thompson, Jasmine Pipella, Guy A. Rutter, Herbert Y. Gaisano and Pere Santamaria. (2023). Islet autoimmunity in human type 1 diabetes: initiation and progression from the perspective of the beta cell. Diabetologia PMID:37488322
Gabriel Brawerman, Vasilis Ntranos and Peter J. Thompson. (2022). Alpha cell dysfunction in type 1 diabetes is independent of a senescence program. Frontiers in Endocrinology PMID:36277717
Gabriel Brawerman, Jasmine Pipella and Peter J. Thompson. (2022). DNA damage to β cells in culture recapitulates features of senescent β cells that accumulate in Type 1 Diabetes. Molecular Metabolism PMID:35660116
See the full list of Dr. Thompson's peer-reviewed journal publications here.